Topic/Category
Year
Topic/Category
Year
20 February 2025
Front-of-pack labelling has become increasingly common in many countries in recent years. For instance, in Chile, nutrition labels serve as warnings and appear only if a food is high in salt, sugar, or fat. Packaged foods in Chile now feature a stop sign label on the front to indicate high levels of these ingredients.
Last month, the U.S. Food and Drug Administration (FDA) proposed a mandate for a front-of-pack nutrition label for most packaged foods. This initiative aims to help consumers identify healthier food options. The FDA has opened a feedback period that will last until May 16, 2025. Once finalised, manufacturers with annual sales of $10 million or more will have three years to comply, while smaller businesses will have four years.
The proposed labels will indicate whether a packaged food or drink contains low, medium, or high levels of sodium, added sugar, and saturated fat, in addition to detailing the percentage of the daily recommended value for these nutrients. Importantly, the new front-of-package label will not replace the existing nutrition facts label on the back; rather, it is designed to complement it. See Figure 1
The FDA believes consumers could use the new front-of-package label to easily compare the healthfulness of different foods at a glance—for example, by examining two yogurt packages to determine which one contains less added sugar.
This initiative is part of the U.S. government’s efforts to address chronic diseases, such as heart disease, diabetes, and cancer, which are leading causes of death and disability in the country.
In 2023, the FDA conducted a study with 10,000 U.S. adults to determine which labels helped consumers make quicker and more accurate assessments of products based on levels of saturated fat, sodium, and added sugars.
The study involved two tasks. In the first task, participants examined three different nutrient profiles of a single front-of-pack scheme and were asked to select the most and least healthy nutrient profiles. Each scheme displayed information about saturated fat, sodium, and added sugars in three different design formats: Guideline Daily Amount (GDA), Nutrition Info, and High In.
In the second task, participants viewed a front-of-package scheme that varied nutrient profiles across three different food products (cereal, frozen entrée, canned soup) and answered questions regarding the product’s healthfulness and nutrient content. Participants could see the entire product label, including the front-of-package information.
Overall, both the GDA and High In schemes did not perform as well as the Nutrition Info schemes in tasks related to understanding the displayed nutrient content. Furthermore, the High In schemes performed worse than both the GDA and Nutrition Info schemes in all attitude and perception measures, except for one measure, “simple to complex,” where there were no significant differences among the three scheme categories.
At home, claims are regulated under the FSANZ Food Standards Code that includes Standard 1.2.7 Nutrition, health and related claims and Schedule 4 – Nutrition, health and related claims. The claim ‘healthy’ is not a prescribed claim under Schedule 4. If a food business were to make a claim that a product is ‘healthy’, the business would be required to have information to support this claim, if asked by an enforcement agency.
The ACCC takes a dim view of false and misleading impressions or claims. Any information or claim that a business provides about its products or services must be accurate, truthful, and based on reasonable grounds. This includes images and descriptions of what is offered, claims about the value, benefits, qualities or performance of products and services. This rule applies to any communication by a business, including through advertising, product packaging, social media, and websites or any other platform.
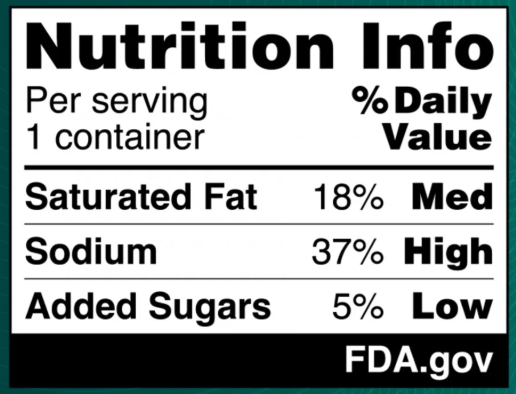
Figure 1